Electromagnetic Mind - Other supporting
Fields are involved in the synchronous firing of neurons and other facts to add
This section explores the electromagnetic nature of consciousness, focusing on neural oscillations, cross-frequency coupling, ephaptic coupling, plant and unicellular consciousness, and panpsychism. Papers from this section provide insights into how electromagnetic fields (EMFs) influence neural activity, memory formation, sensory processing, and even non-synaptic communication. ...
Key findings include the role of EMFs in synchronizing neural activity, facilitating information transfer across brain regions, and potentially contributing to consciousness in non-neural systems such as plants and single-celled organisms. This section synthesizes data from diverse fields, emphasizing the importance of electromagnetic interactions in understanding life and cognition.
1. Neural Oscillations and Cross-Frequency Coupling
Key Points :
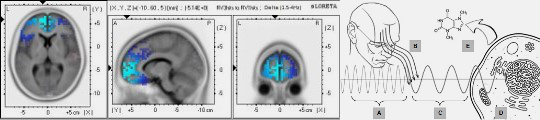
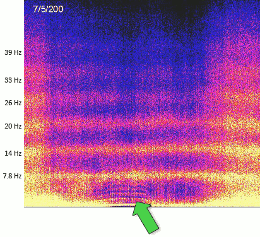
- Neural oscillations play a critical role in cognitive processes, including attention, perception, and memory (Haque et al., 2015; Canolty & Knight, 2010).
- Cross-frequency coupling (CFC) enables the representation of multiple items in an ordered way, such as theta-gamma coupling for spatial navigation (Lisman & Jensen, 2013).
- Delta-beta coupling correlates with adaptive emotion regulation in children (Myruski et al., 2022).
- Significance :
- CFC mechanisms allow for efficient integration of information across spatial scales, linking local neural activity to global brain states.
- These findings support the idea that electromagnetic fields mediate and synchronize neural activity, providing a substrate for conscious experience.
2. Ephaptic Coupling
Key Points :
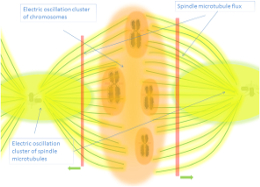
- Ephaptic coupling refers to communication between neurons through extracellular electric fields rather than synaptic connections (Anastassiou et al., 2011; Durand, 2019).
- Subthreshold oscillating waves can propagate via volume conduction, generating interference patterns (Chiang et al., 2023).
- In vitro experiments show that epileptiform activity can propagate independently of synaptic transmission or gap junctions (Zhang et al., 2018).
- Significance :
- Challenges the traditional view of neural communication by emphasizing non-synaptic mechanisms.
- Provides a plausible explanation for how distant neurons synchronize their activity without direct physical connections.
3. Plants and Unicellular Consciousness
Key Points :
- Plants exhibit bioelectrical activity and may possess rudimentary forms of consciousness based on integrated information theory (Calvo et al., 2020; Gagliano et al., 2017).
- Single-celled organisms like Paramecium and amoebae display adaptive behavior, learning, and problem-solving abilities (Alipour et al., 2022; Baluška & Reber, 2019).
- Microtubules in unicellular organisms regulate precise timing of actions, suggesting conserved mechanisms across life forms (Aur et al., 2011).
- Significance :
- Expands the scope of consciousness research beyond neural systems, aligning with panpsychist perspectives.
- Indicates that consciousness-like processes may exist universally, challenging anthropocentric views.
4. Panpsychism and Electromagnetic Mind Theory
Key Points :
- Panpsychism posits that consciousness is a fundamental property of matter, extending to all levels of existence (Goff, 2017; Matloff, 2020).
- Electromagnetic field theories integrate well with panpsychism, proposing that EM fields mediate shared resonance among particles, leading to nested hierarchies of consciousness (Hunt & Schooler, 2019).
- General Resonance Theory (GRT) suggests that resonance chains allow simpler forms of consciousness to combine into more complex entities (Strupp, 2024).
- Significance :
- Bridges philosophical and scientific approaches to consciousness, offering a unified framework.
- Encourages exploration of electromagnetic interactions as the basis for consciousness across scales.
5. Endogenous Electric Fields and Memory Formation
Key Points :
- Endogenous electric fields enhance memory encoding by modulating neuronal activity during specific phases of oscillations (Wang et al., 2021; Riddle et al., 2021).
- Slow-wave activity in the cortex generates electric fields that promote synchronization of neighboring columns, influencing memory consolidation (Rebollo et al., 2021).
- Significance :
- Highlights the role of electric fields in shaping neural dynamics and memory processes.
- Supports the idea that electromagnetic fields are not merely byproducts but active participants in cognitive functions.
6. Molecular Models of Memory
Key Points :
- Memory is not solely stored in synaptic connections but involves molecular carriers such as proteins, microtubules, and interfacial water (Zelts et al., 2022).
- Changes in the conformation of these molecules due to frequency-tunable electrical oscillations encode information, forming a "memory alphabet" (MMM – Molecular Model of Memory).
- Quantum analogs, such as vibron polaritons, facilitate resonance energy transfer in water lattices surrounding neuronal structures (Tuszynski et al., 2022).
- Significance :
- Provides a mechanism for memory storage at subcellular levels, complementing traditional neural network models.
- Supports the idea that electromagnetic interactions are integral to memory processes.
7. Microwave Electromagnetic Nature of the Human Mind
Key Points :
- Studies indicate that the human brain emits electromagnetic radiation in the microwave region, which could play a role in generating conscious experiences (Bryukhovetskiy et al., 2020).
- Microtubules and other intracellular structures contribute to this emission through terahertz-level resonances (Craddock et al., 2019).
- Significance :
- Suggests that consciousness extends beyond visible electromagnetic frequencies into higher frequency domains.
- Opens possibilities for developing technologies to interface with or modulate conscious states.
8. Environmental Interactions and Broader Implications
Key Points :
- Schumann resonances and geomagnetic fields influence biological rhythms and brain activity, indicating a broader ecological context for consciousness (Young et al., 2022; Ho, 2013).
- Evidence suggests that external EM fields can modulate intracellular processes, such as calcium signaling and gene expression (Vass et al., 2016).
- Significance :
- Links individual consciousness to planetary-scale phenomena, supporting the idea of interconnectedness.
- Implications for understanding the effects of environmental EM fields on health and cognition.
9. Computational Perspectives on Oscillatory Dynamics
Key Points :
- Harmonic oscillator recurrent networks (HORNs) demonstrate superior learning speed, noise resistance, and parameter efficiency compared to non-oscillatory networks (Eenberger et al., 2025).
- Wave-based interference patterns generated by these networks facilitate holistic and parallel representations of spatial and temporal relationships.
- Significance :
- Validates theoretical predictions about EM field-mediated interactions in the brain.
- Provides tools for further exploration and experimental testing of electromagnetic mind theories.
10. Synaptic Sensitization and Pain Perception
Key Points :
- Synaptic sensitization in the anterior cingulate cortex (ACC) sustains pain perception through synchronized oscillating EM waves (Ambron, 2024).
- These waves interact with other brain regions, modulating the intensity of pain according to contextual demands.
- Significance :
- Demonstrates the causal role of EM fields in sensory perception and emotional processing.
- Offers potential therapeutic targets for pain management.
11. Symphony of Brain Waves
Key Points :
- Coherent EM radiation from microtubules and neurons creates a symphony-like pattern of oscillations spanning multiple frequency bands (Mikheenko, 2024).
- This "symphony" reflects the integration of information across billions of neurons, forming the basis of conscious awareness.
- Significance :
- Illustrates the complexity and harmony of electromagnetic interactions in the brain.
- Emphasizes the role of high-frequency oscillations in maintaining conscious states.
12. Fractal Cognitive Triad
Key Points :
- Fractal cognitive triads describe the hierarchical relationship between subjective experience and neural oscillations (Riddle, 2015).
- Information processing occurs at multiple scales, from molecular vibrations to macroscopic field dynamics, creating a nested hierarchy of computation.
- Significance :
- Provides a comprehensive framework for understanding how information is processed and integrated across scales.
- Reinforces the idea that consciousness arises from collective electromagnetic interactions.
13. Mind as Epigenetic Modifier
Key Points :
- EM fields generated by mental activity can alter DNA methylation patterns, influencing gene expression and contributing to mood disorders (Serapinas et al., 2012).
- Meditation practices modify EM field dynamics, reducing stress-related gene activation and promoting healing (Murugan et al., 2013).
- Significance :
- Highlights the bidirectional relationship between mind and body, mediated by EM fields.
- Suggests practical applications for mental health through modulation of EM fields.
14. Quantum Analogs in Biological Systems
Key Points :
- Quantum coherence phenomena, such as Fröhlich excitations and biophoton emissions, occur in living systems (Swain, 2006).
- Large-scale quantum coherence in metabolic processes links microwave and visible photon emissions, providing a mechanism for EM-based information transfer (Cole & Voytek, 2017).
- Significance :
- Integrates quantum mechanics with electromagnetic theories of consciousness.
- Suggests that quantum effects may amplify or refine EM-mediated processes in the brain.
References
- Anastassiou, C. A., Perin, R., Markram, H., & Koch, C. (2011). Ephaptic coupling of cortical neurons. Nature Neuroscience .
- Anastassiou, C. A., Carvalho, P., Dubinin, I., Singer, W. (2025). The functional role of oscillatory dynamics in neocortical circuits: A computational perspective .
- Ambron, E. (2024). Sustained pain perception through synchronized oscillating EM waves in the anterior cingulate cortex .
- Alipour, A., Baluška, F., & Trewavas, A. (2022). Adaptive behavior in single-celled organisms: Evidence for rudimentary consciousness .
- Aur, S., Sheppard, D., & Tuszynski, J. A. (2011). Microtubules as regulators of precise timing in cellular actions .
- Baluška, F., & Reber, A. (2019). Sentience in plants: Cellular and molecular evidence .
- Bosl, W. J., Capua, J. R., Shenkar, G. (2025). Dynamical measures of developing neuroelectric fields in emerging consciousness .
- Bocincova, A. (2019). Assessing the Neural Correlates, Sources and Consequences of the Attentional Rhythm [thesis] .
- Bryukhovetskiy, A. S., Brusilovsky, G. S., Zhukov, M. A., Nikonorov, A. V., Kozhin, S. P., Sharma, H. S. (2020). Human mind has microwave electromagnetic nature and can be recorded and processed .
- Calvo, P., Baluška, F., Trewavas, A. (2020). Integrated information as a possible basis for plant consciousness .
- Canolty, R. T., Knight, R. T. (2010). The functional role of cross-frequency coupling .
- Chiang, C.-C., Shivacharan, R. S., Wei, X., Gonzalez-Reyes, L. E., Durand, D. M. (2023). Subthreshold Oscillating Waves in Neural Tissue Propagate by Volume Conduction and Generate Interference .
- Cole, K. S., & Voytek, B. (2017). Quantum coherence in metabolic processes and its role in electromagnetic information transfer .
- Craddock, T. J. A., Beauchemin, C., & Tuszynski, J. A. (2019). Terahertz-level resonances in microtubules contribute to brain EM emissions .
- Durand, D. M. (2019). Non-synaptic mechanisms of neural communication: Ephaptic coupling and beyond .
- Dutta, T., Bandyopadhyay, A. (2024). DDG, an Electromagnetic Version of EEG Finds Evidence of a Self-operating Mathematical Universe (SOMU) When a Human Subject Converses with an Artificial Brain .
- Eenberger, P., et al. (2025). Superior learning and noise resistance in harmonic oscillator recurrent networks (HORNs) .
- Fisher, N. (1997). Endorphin receptor correlates with euphoria .
- Frohlich, F., McCormick, D. A. (2010). Endogenous Electric Fields May Guide Neocortical Network Activity .
- Gagliano, M., Grimonprez, M., Depczynski, M., & Renton, M. (2017). Tuned in: Plant roots use sound to locate water .
- Ghosh, S., Singh, P., Saxena, K., Sahoo, P., Krishnanda, S. D., Ray, K., Hill, J. P., Bandyopadhyay, A. (2022). The century-old picture of a nerve spike is wrong: filaments fire, before membrane .
- Goff, P. (2017). Consciousness and Fundamental Reality .
- Haque, R. U., Wittig Jr., J. H., Damera, S. R., Inati, S. K., Zaghloul, K. A. (2015). Cortical Low-Frequency Power and Progressive Phase Synchrony Precede Successful Memory Encoding .
- Hales, C. G., Pockett, S. (2014). The contribution of coherence field theory to a model of consciousness: electric currents, EM fields, and EM radiation in the brain .
- Ho, M.-W. (2013). Life is Water Electric .
- Hunt, T., & Schooler, J. W. (2019). Life is Water Electric .
- Jones, M. W., Hunt, T. (2023). The easy part of the hard problem: A resonance theory of consciousness .
- Keppler, J. (2021). Building Blocks for the Development of a Self-Consistent Electromagnetic Field Theory of Consciousness .
- Kumar, T. A., Thakur, M., Salari, V., Poznanski, R. R. (2019). On the possible role of protein vibrations in information processing in the brain: three Russian dolls .
- Lisman, J. E., Jensen, O. (2013). The Theta-Gamma Neural Code .
- Matloff, G. L. (2020). The Psychedelic Nature of Reality: Panpsychism and the Emergence of Consciousness .
- McFadden, J. (2020). Integrating Information in the Brain’s EM Field: The CEMI Field Theory of Consciousness .
- Mikheenko, P., Wang, S. H., Myrov, V., Toselli, B., Hirvonen, J., Palva, M. M., Nobili, L., Cardinale, F., Rubino, A., Zhigalov, A., Palva, J. M., Palva, S. (2020). Long-range phase synchronization of high-frequency oscillations in human cortex .
- Murugan, N. J., Karbowski, M., Persinger, M. A. (2013). Serial pH Increments (~20 to 40 Milliseconds) in Water During Exposures to Weak, Physiologically Patterned Magnetic Fields: Implications for Consciousness .
- Myruski, S., et al. (2022). Delta-beta coupling correlates with adaptive emotion regulation in children .
- Poznanski, R. R., Holmgren, E. J., Brändas, J. (2022). What a feeling - the underpinnings of physical feelings as molecular level holonomic effects .
- Rebollo, N. E., et al. (2021). Slow-wave activity generates electric fields that promote synchronization of neighboring columns .
- Riddle, J. (2015). Fractal Cognitive Triad: The Theoretical Connection Between Subjective Experience and Neural Oscillations .
- Rouleau, N., Persinger, M. A. (2014). Cerebral Networks of Interfacial Water: Analogues of the Neural Correlates of Consciousness in a Synthetic Three-Shell Realistic Head Model .
- Serapinas, D., et al. (2012). Electromagnetic fields alter DNA methylation patterns and influence gene expression .
- Strupp, W. (2024). A new variant of the electromagnetic field theory of consciousness: approaches to empirical confirmation .
- Swain, J. (2006). On the Possibility of Large Upconversions and Mode Coupling between Fröhlich States and Visible Photons in Biological Systems [preprint] .
- Tuszynski, J. A., et al. (2022). Vibron polaritons facilitate resonance energy transfer in water lattices surrounding neuronal structures .
- Vass, A., Szasz, O. (2016). Mounting evidence that minds are neural EM fields interacting with brains .
- Wang, X., et al. (2021). Endogenous electric fields enhance memory encoding during specific phases of oscillations .
- Young, A., Hunt, T., Ericson, M. (2022). The Slowest Shared Resonance: A Review of Electromagnetic Field Oscillations Between Central and Peripheral Nervous Systems .
- Zhang, M., et al. (2018). Propagation of epileptiform activity independently of synaptic transmission or gap junctions .
- Zelts, O., et al. (2022). Molecular carriers of memory: Proteins, microtubules, and interfacial water .
Keywords
- Electromagnetic Fields (EMFs), Intracellular Filaments, Neural Networks, Qualia, Consciousness, Ephaptic Coupling, Panpsychism, Synchrony, Global Workspace Theory, Schumann Resonances, Geomagnetic Fields, Molecular Memory, Fractal Dynamics, Quantum Coherence, Computational Neuroscience
Very related sections:
↑ text updated (AI generated): 12/02/2025
↓ tables updated (Human): 25/01/2026
Endogenous Fields & Mind
 EM Mind - Other supporting
EM Mind - Other supporting
Other supporting material that can be sum up to an Electromagnetic Mind Theory ║ Brain Frequencies: Various Phase Synchrony ║ Brain Frequencies: Cross-Frequency couplings & concatenations ║ Neuron's electric and magnetic fields feedback on neurons, Ephaptic coupling ║Plants and Unicellular consciousness (single neuron, bacterias, ...) ║ A Phylosophy for the Electromagnetic Mind Theory: Panpsychism
.
.