Electromagnetism & Morphogenesis
Fields guiding the positioning of organelles in cells, cells in organs and organs in bodies
It’s being discovered that electromagnetic (EM) fields have a lot of to do with the creation and maintenance of form in biological systems, and that they work in all scales, from cellular to body, guiding morphogenesis. This is consistent with an electromagnetic mind theory that also equate mind to the sense of be alive, as those fields operate also at unicellular scales. ...
This last is important by principle, as it provide us with a powerful explanatory tool for the intelligence and memory capabilities of unicellular the entities [1] or the non nervous biological systems including plants.
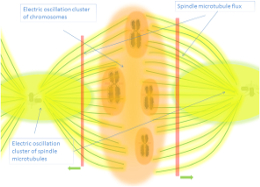
In this sense there are especially valuable the works of Pietak [2][3], although they are theoretical and no experimental, where the three dimensional architectures of developing plant flower buds show striking parallels with the resonance patterns that electromagnetic energies can form and where, curiously, the two components of the EM fields (electric and magnetic) are interchangeable but always representing opposite position/function like the seeds on one side and external walls on the other [2]:
" Similar to the abutilon ovary and squash male flower bud, cells in the female squash ovary in regions that correlate to highest electric field strength have changed into placental tissue cells, while the six places of high magnetic field strength are also the six places where ova form in the squash ovary."
" Overall, the structural evidence and physical uniqueness of EM mode patterns indicates developing plant organs can support EM resonances, whereby the electric and magnetic field components guide symmetry-breaking and therefore resemble the first pattern to emerge in primordia. Rich in positional information, the EM resonant mode represents a possible physical manifestation of the morphogenetic field."
It is experimentally proven that in the case of the flowers of the plants those fields extends beyond the boundaries of them [4] and is used by pollinators as a detection/informative method [5].
But returning to a more morphogenesis centered perspective it is a growing body of evidence that electric currents guide the migration of cells, for example Cao et al. [6] reported electric field dependent migration of neuroblasts, and as reviewed by Levin et al. [7] specific voltage range is necessary for, for example, the demarcation of eye fields in the frog embryo and, artificially setting other somatic cells to the eye-specific voltage range, provoke the formation of eyes in aberrant locations. Moreover those can include tissues that are not in the normal anterior ectoderm lineage: eyes could be formed in the gut, on the tail, or in the lateral plate mesoderm.
The disruption of the spatial gradient of the transmembrane potential (Vmem) of the cells lining the neural tube diminishes or eliminates the expression of early brain markers, and causes also an anatomical mispatterning of the brain [8]. Electric fields are not only capable of guide cells to everywhere and alter the final form but also are capable to reverse morphogenesis [9]:
" We show that an external electric field above a critical amplitude can halt and even reverse the course of morphogenesis in whole-body Hydra regeneration on demand. The reversal trajectory maintains the integrity of the tissue and its regeneration capability. We further show that these reversal dynamics are induced by enhanced electrical excitations of the tissue. It demonstrates that electrical processes play an instructive role in morphogenesis to a level that can direct developmental trajectories, commonly thought to be forward-driven programmed biochemical processes."
But as an important result in this experiment is that also has confirmed the importance of the frequency of the electric field and the involvement of calcium activity:
" These data demonstrate the existence of a frequency cutoff around 1 kHz, above which the elevated Ca2+ activity is reduced, and morphogenesis is restored from its suspended state. This upper frequency cutoff is not a sharp cutoff at precisely 1 kHz. Nevertheless, further experiments demonstrate that at frequencies higher than ∼1 kHz, the regeneration process is insensitive to the external electric field."
Which evidences that the phenomenon is an electrodynamic dependent one, and no mere an electrostatic phenomenon. Magnetic fields are also always present, for example in [10] there has been detected endogenous magnetic fields arising from the ion transport/movement through the cell membrane.
In [11] Authors develop an ion channel-based model that describes multicellular states on the basis of spatio-temporal patterns of electrical potentials in aggregates of non-excitable cells. And try to give answers to various questions that include how can a single-cell contribute to the control of multicellular ensembles based on the spatio-temporal pattern of electrical potentials or how can oscillatory patterns arise from the single-cell bioelectrical dynamics.
The relationship of ion oscillations with morphogenesis is also exemplified in [12] where there is observed the dynamic changes of bioelectric currents in developing chicken embryos:
" Before feather bud elongation, EF endogenous to dorsal skin was relatively homogenous and exhibited inward directionality. At the onset of elongation, outward electric current emerged at the anterior side of each feather bud, implying a heterogenization of the EF into multiple smaller electric circuits. Tissue-wide long-range Ca 2+ oscillations were observed in bud mesenchyme. Dampening these oscillations or introduction of exogenous oscillations altered feather morphology. Feather mesenchymal cell movement changes direction markedly when voltage-gated Ca 2+ channels (VGCCs) or gap junctions were inhibited."
It must be taken in consideration that the collective oscillations of Ca2+ ions can generate endogenous electromagnetic fields and that there are serious indications that there is information encoded both in the amplitude modulation and in the frequency modulation of Ca2+ oscillations [13]:
" Decoding is used by the cell to interpret the information carried by the Ca2 + oscillation []. This information deciphering occurs when one or several intracellular molecules sense the signal and change their activities accordingly. The process is similar to electromagnetic radiation being received by an antenna on a radio and translated into sound. Mathematical modeling of a generic Ca2 + sensitive protein has shown that it is possible to decode Ca2 + oscillations on the basis of the frequency itself, the duration of the single transients or the amplitude."
Wells [14] focused on plasma membrane patterns that generate endogenous electric fields that can provide three-dimensional coordination systems for embryo development. In a review by Funk [15] he distinguishes two aspects of this ambit: low magnitude membrane potentials and related electric fields (bioelectricity), and cell migration under the guiding cue of electric fields. He described for example how in osteoblasts, the directional information of electric fields is captured by charged transporters on the cell membrane and transferred into signaling mechanisms that modulate the cytoskeletal and motor proteins, resulting in a persistent directional migration along an electric field guiding cue.
Among others, electric fields activate a number of channels and that variations in the extracellular and intracellular environment as well as the distribution of channels on the membrane contribute to the galvanotactic response [16]. Michael Levin in [17] mentioned:
" The genome is tightly linked to bioelectric signaling, via ion channel proteins that shape the gradients, downstream genes whose transcription is regulated by voltage, and transduction machinery that converts changes in bioelectric state to second-messenger cascades. However, the data clearly indicate that bioelectric signaling is an autonomous layer of control not reducible to a biochemical or genetic account of cell state."
Also Michael Levin, and Maria Lobikin, realized a review [18] where are well described some of the issues related to endogenous bioelectric currents, just as they wrote in the abstract:
" Complex pattern formation requires mechanisms to coordinate individual cell behavior towards the anatomical needs of the host organism. Alongside the well-studied biochemical and genetic signals functions an important and powerful system of bioelectrical communication. All cells, not just excitable nerve and muscle, utilize ion channels and pumps to drive standing gradients of ion content and transmembrane resting potential. In this chapter, we discuss the data that show that these bioelectrical properties are key determinants of cell migration, differentiation, and proliferation. We also highlight the evidence for spatio-temporal gradients of transmembrane voltage potential as an instructive cue that encodes positional information and organ identity, and thus regulates the creation and maintenance of large-scale shape. In a variety of model systems, it is now clear that bioelectric prepatterns function during embryonic development, organ regeneration, and cancer suppression."
Electric fields, magnetic fields and electromagnetic fields can determine how cells move and adhere to surfaces; how the migration of multiple cells are coordinated and regulated; how cells interact with neighboring cells, and also be associated to changes in their microenvironment [19].
Moreover, as pointed in the subtitle of this section internal organelles and structures of cells may be also disposed following what the internally generated fields dictate, in this sense, for example, in [20] it has been found that intracellular pH and membrane potential changes simulate bioelectrical changes occurring naturally and leading to the cytoskeletal modifications observed during differentiation of the follicle-cell epithelium. Therefore, gradual modifications of electrochemical signals can serve as physiological means to regulate cell and tissue architecture by modifying cytoskeletal patterns. More generally in [21] are exposed the latest findings on the electroconductive properties of cellular internal components, and of course, when it speak about electrical conduction it can also speak about sensitivity to external and internal electromagnetic fields.
References:
Very related sections:
↑ text updated: 08/07/2020
↓ tables updated: 15/07/2023
Endogenous Fields & Mind
 EM & Morphogenetics
EM & Morphogenetics
.
.