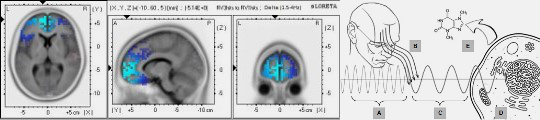
Electromagnetism & Microtubules
Those cellular constituents resonate electrically and generate information fields
Microtubules are essential cellular biopolymers that have much more than a structural and cytoeskeletar function, in the same way as the majority of biological molecules and structures they are electrically polar, vibrational synchronizations capable of generate electromagnetic fields are observed in different microtubule group topologies or also in isolated units. ...
Microtubules are the main constituents of the cellular cytoskeleton together with microtubule associated proteins (MAPs), intermediary filaments and actin filaments, and they are specially abundant on neurons. The main characteristics of microtubules are well described in various of the papers listed in the table, here only there will be pointed out some of their feasible relationship with endogenous electromagnetic fields (and and by extension with an electromagnetic mind theory).
Theoretical studies of the microtubule structure have been disclosed their vibrational normal modes in a wide frequency range from acoustic to GHz frequencies, but here we will follow for now the main theory presented in this website, because of it’s capacity to integrate various frequencies resonantly implying different scales, so for example we can see that Sahu et al. [1] have controlled the grow of microtubules and also its constituents protein (tubulin) shape controlling them electromagnetically with certain resonant frequencies:
" We report remarkable observation that the pristine tubulins form the cylindrical shape without GTP molecule even in solution using particular resonance frequency of tubulin (Some resonance peaks for tubulins are: [37,46, 91, 137, 176, 281, 430] MHz; [9, 19, 78, 160, 224] GHz; [28, 88, 127, 340] THz. Some microtubule resonance peaks are: [120, 240, 320] kHz; [12,20, 22, 30, 101, 113, 185, 204] MHz; [3, 7, 13, 18] GHz."
And the idea is that in a particular protein (tubulin for example) the electromagnetic and mechanical oscillations have a common time or frequency region where, both electromagnetic and mechanical oscillations merge, so one can be manipulated with another. Although in the paper mentioned above they present experimental results, there is also one interesting theoretical prediction of resonant frequencies of microtubules and tubulins made by Cosic et al. [2] and based on the Resonant Recognition Model (RRM), a model that have its own dedicated section on this website [3].
It has been found experimentally [4] that a collective terahertz vibration in microtubule’s tubulin dimmers exists that can be used to long range recognition of the appropriate polymerization rates (and since microtubule stiffness variation can affect whole cell morphology and intracellular transport, this could lead to changes in the timing of neuron firing and neuron function) that could be ‘masked’ if anesthetic molecules are added.
There is very probably a relation between the electrical properties of microtubules and the generation of action potential in neurons, a cue for this can be found also in [5] where a solution with an widout microtubules is exposed to electrif fields of different frequencies and is found that while addition of both MTs and tubulin generally led to an increase in solution resistance in the frequency range of 1-20 HZ the resistence began to dismis at higher frequencies, with a peak between 20 and 60 Hz, so there is a an increase in solution conductance which authors have been hypothesised to arise from oscillatory ionic movement across the MT lattice through nanopores formed between adjacent tubulin dimers, as the authors are aware of:
" It is worth nothing that this region falls within the gamma frequency regime (20-60 Hz), implicating such quasi-resonant phenomena as a possible explanation for the source of low frequency intraneuronal electrical oscillations."
In [6] authors applied the patch clamp technique to two-dimensional Brain Microtubule sheets, to characterize their electrical properties and found that they generated cation-selective oscillatory electrical currents with a prominent a fundamental frequency at 29 Hz. (and as the explain several biological oscillatory phenomena display around 30 Hz cycles). A posterior investigation from the same group [7] but using in this case bundles of microtubules also revealed that they spontaneously generate electrical oscillations and bursts of electrical activity similar to action potentials with a prominent fundamental frequency at 39 Hz, that progressed through various periodic regimes.
The microtubule itself can also generate electromagnetic fields in the low frequency range, in [8] a model to provide a realistic picture of the localization of energy in microtubules is created:
"... We establish that the electromechanical vibrations in MTs [microtubules] can generate an electromagnetic field in the form of an electric pulse (breathers) which propagates along MT serving as signaling pathway in neuronal cells. The DBs [discrete breathers] in MT can be viewed as a bit of information whose propagation can be controlled by an electric filed. They might perform the role of elementary logic gates, thus implementing a subneuronal mode of computation. The generated DBs present us with novel possibilities for the direct interaction between the local electromagnetic field and the cytoskeletal structures in neurons. Thus, we emphasize that the effect of discreteness and electric field plays a significant role in Mts."
An experimental setup to verify the possibilities of wireless information transfer between microtubules has been develeped in [9] where authors build up artificial microtubules like devices having analogous characteristics to demonstrate that wireless magnetic communication (not electromagnetic) between neighboring microtubules is feasible, moreover the found that those microtubules harvest energy from the noise present in the environment to achieve communication.
They demonstrate that the device (that is electronically useless, without the ability to transmit electron) converts noise of a particular type into an ordered signal, a magnetic signal that includes information of phase, and then the signal rebuilds in the sender.
Anyways they do not try to emulate the special role of water in microtubules, the water crystal located at the core of the microtubular structure that is the channel by which microtubules acquire their remarkable electronic features, a we will see.
On the other side, Georgiev et al. [10] predicted that the local electromagnetic fields support information that could be converted into specific protein tail tubulin conformational states, and in their paper include concepts like water order in the microtubule cavity and biophotonic emissions:
" Among the long-range order creating phenomena induced by the interaction between the water dipoles and the local electromagnetic field we may find a specific one in which the collective dynamics of the water electric dipole (WEDP) field in the spatial region of linear dimension up to 50 µm can give rise to a cooperative emission of coherent photons with induced energy by certain systems external to the quantum system of the electromagnetic field and the WEDP field."
As Pokorny et al. [11] reviews:
" Organization of bodies with macroscopic dimensions and synchronization of mutually dependent activity requires forces of corresponding extension. Excitation of coherent electromagnetic (EMG) field is considered to be an essential mechanism of biological functions. .. Sahu et al. [5, 6] measured resonant frequencies of isolated microtubules in the classical frequency range below 20 GHz, in the far infrared region in the range of 300–1500 cm−1 , and the UV absorption-emission spectrum. The frequency spectrum from 20 GHz to 100 GHz should be also analyzed."
In regard to the ultraviolet spectrum and microtubules we can speak about "biophotons and microtubules", and in this topic (and their relation to brain activity) there is an entire section on this site [12].
For example Nistreanu [13] described an hypothesis (also outlined in other papers) where...
" Microtubules are considered as quantum cavities. Their role is to provide a single mode of biophoton field, in such a way that water molecules to be considered not as independent individuals, but rather as whole, in this manner water molecules are embedded in and interacting with a common radiation field. In the model proposed, collective behavior of water molecules is characterized by coherent water states analogous to Bloch states, whose main feature is to trap biophotons in a collective fashion."
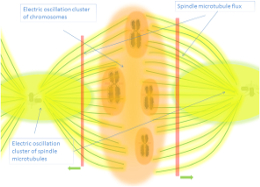
Returning to current section, it can be mentionable also the idea proposed by Zhao [14] where centrioles, that are formed by one microtubule in the center and nine triplicate around connected by motor proteins, functions as molecular dynamo rotating around the central micotubule:
" The rotation and electric oscillation of each centriole will generate a dynamic electromagnetic field that mimic the physical structure of the centriole, and the orthogonal arrangement of centrioles of each centrosome will result in the microtubules of the barrel structure of each centriole to cut the electromagnetic field generated by the other centriole when rotating (Figure 1). Such a natural design makes centrosome to function as a molecular dynamo, generating directional electron flow through the dipolar structure of each individual microtubule in the centrosome, transforming the energy from ATP to electric current."
In any case, as mentioned at the initial paragraph, electrical oscillations are also detected in isolated microtubules [15]:
" Recent in vitro electrophysiological studies indicate that different brain MT structures, including two-dimensional sheets and bundles, generate highly synchronous electrical oscillations. However, no information has been heretofore available as to whether isolated MTs also engage in electrical oscillations. In the present study, a broader spectrum of fundamental frequencies was always observed in isolated MTs as compared to the MT sheets. This interesting finding is consistent with the possibility that more structured MT complexes (i.e. bundles, sheets) may render more coherent response at given oscillatory frequencies and raise the hypothesis that combined MTs may tend to oscillate and entrain together."
So this is absolutely in accordance with a theory of resonant electromagnetic field along all scales of biological system that is related to mind, and where microtubules as could not be otherwise play an important role.
References:
Very related sections:
↑ text updated: 06/07/2020
↓ tables updated: 31/07/2021
Endogenous Fields & Mind
 EM & Microtubules
EM & Microtubules
.
.